Glioblastoma Neuroimaging
Neuroimaging plays a central role in the initial diagnosis and subsequent monitoring of multimodal therapies offered to patients with glioblastoma – the most malignant type of brain cancer. The authors present a comprehensive description of current state-of-the-art clinical neuroimaging for glioblastoma. They cover the basic concepts and most recent applications of clinical structural neuroimaging, as well as the latest innovations and up-and-coming techniques for functional imaging of glioblastoma patients.
Glioblastoma (GBM) is the most common adult primary intracranial neoplasm, accounting for 15% of all intracranial neoplasms and approximately 50% of all astrocytomas. GBMs are high-grade astrocytomas; they are therefore generally aggressive, largely resistant to therapy, and have a corresponding poor prognosis. They have a predilection for spreading along the condensed white matter tracts such as corticospinal tracts and corpus callosum to involve the contralateral hemisphere. Glioblastoma was previously known as glioblastoma multiforme; the multiforme refers to the tumor heterogeneity. The WHO classification has dropped the ‘multiforme’ and thus it is best to refer to these tumors merely as glioblastomas.
Primary glioblastomas are those that arise de novo, without a pre-existing lower grade diffuse astrocytoma. They account for 90% of all glioblastomas and are more aggressive than secondary glioblastomas and they tend to occur in older individuals. Primary glioblastomas are almost invariably IDH wild-type. They tend to have amplification of EGFR and overexpression of MDM2, PTEN mutation and/or loss of heterozygosity of chromosome 10p.
Secondary glioblastomas, in contrast, are those which arise from a pre-existing lower grade diffuse astrocytoma. They are relatively uncommon, only accounting for approximately 10% of all glioblastomas. These tumors tend to be less aggressive than primary glioblastomas and they tend to occur in younger patients. Interestingly, and of uncertain significance, they have a predilection for the frontal lobes. Characteristically, and unlike primary tumors, secondary glioblastomas tend to be IDH mutant (positive), a mutation shared by over 80% of grade II and III astrocytomas. Secondary glioblastomas also demonstrate p53 mutations, amplification of PDGF-A, loss of heterozygosity of chromosomes 10q and 17p, loss of 19q and increased telomerase activity and hTERT expression.
In the current WHO classification of CNS tumors, three glioblastoma histological variants are recognized (which are discussed separately) as well as a number of histological patterns which are discussed below.
The three recognized variants are:
- Giant cell glioblastoma
- gliosarcoma
- Epithelioid glioblastoma
The vast majority of glioblastomas are sporadic. Rarely they are related to prior radiation exposure (radiation-induced GBM). They can also occur as part of rare inherited tumor syndromes, such as p53 mutation related syndromes such as neurofibromatosis type1 (NF1) and Li-Fraumeni syndrome. Other syndromes in which GBMs are encountered include Turcot syndrome, Ollier disease and Maffucci syndrome.
Typically patients present in one of three ways:
- focal neurological deficit
- symptoms of increased intracranial pressure
- seizures
Rarely (<2%) intratumoral hemorrhage occurs and patients may present acutely with stroke-like symptoms and signs.
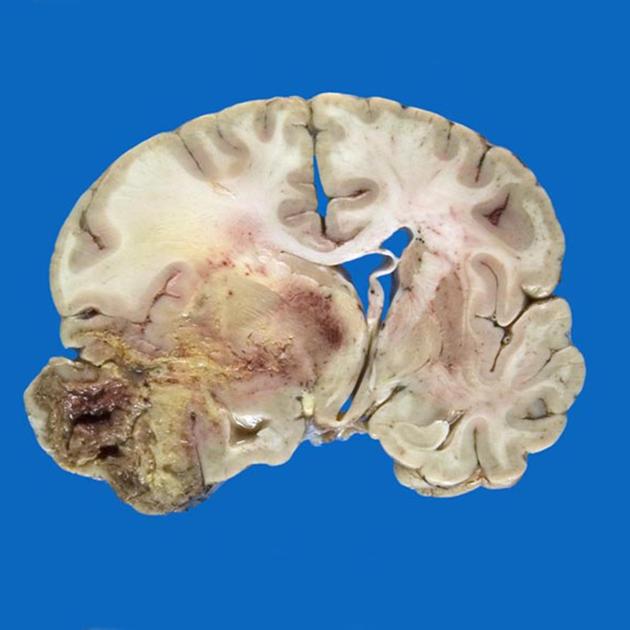
Although glioblastomas can arise anywhere within the brain, they have a predilection for the subcortical white matter and deep grey matter of the cerebral hemispheres, particularly the temporal lobe. Glioblastomas are typically poorly-marginated, diffusely infiltrating necrotic masses localized to the cerebral hemispheres. The supratentorial white matter is the most common location.
These tumors may be firm or gelatinous. Considerable regional variation in appearance is characteristic. Some areas are firm and white, some are soft and yellow (secondary to necrosis), and still others are cystic with local hemorrhage. GBMs have significant variability in size from only a few centimeters to lesions that replace a hemisphere. Infiltration beyond the visible tumor margin is always present. These tumors are multifocal in 20% of patients but are rarely truly multicentric. Glioblastomas are capable of demonstrating varied patterns, sometimes within the one tumor. In addition to the three recognized variants (giant cell glioblastoma, gliosarcoma, and epithelioid glioblastoma) additional histological features are sometimes encountered which impact imaging appearance and biological behavior. Most of these are seen predominantly in primary IDH wild-type glioblastomas.
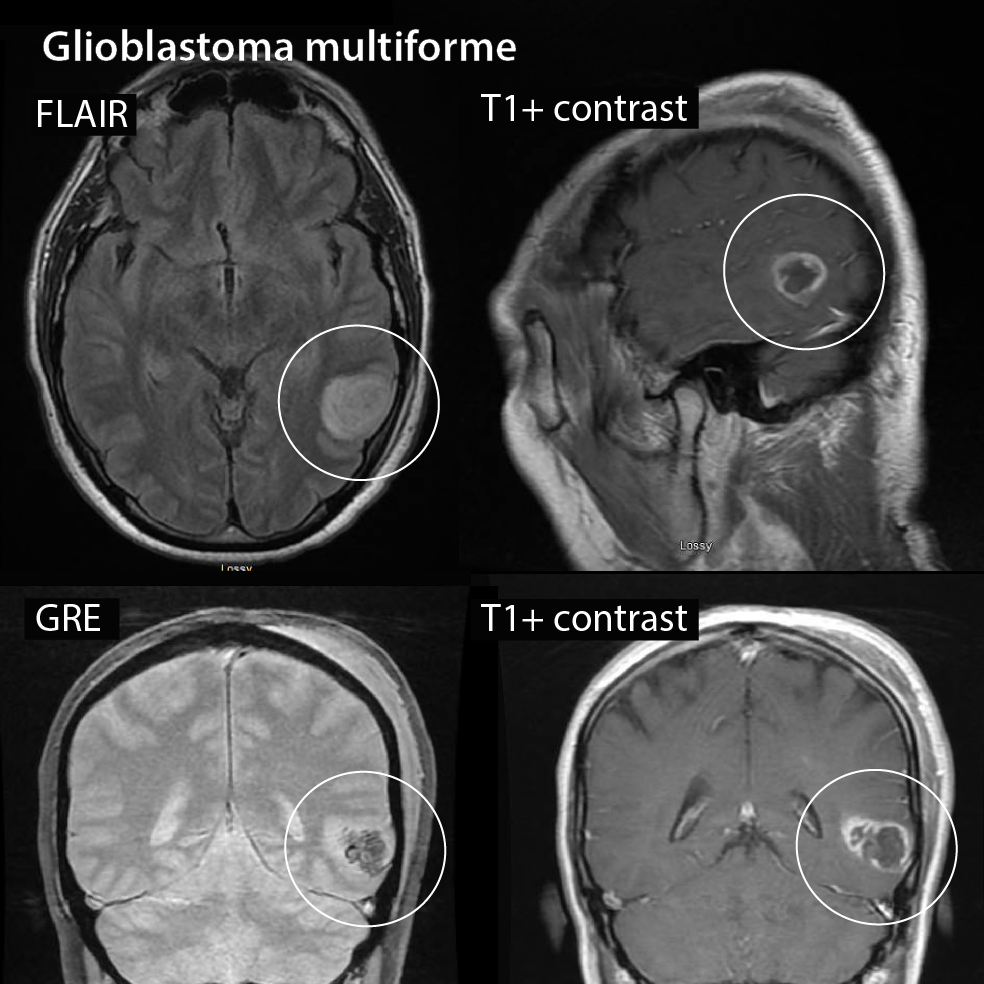
What does a glioblastoma look like on MRI? A low grade glioma or astrocytoma may show only a low density area (dark area) whereas high grade gliomas (Glioblastoma) usually show more contrast enhancement (white on the outside) and necrosis in the middle.
Radiographic features
Glioblastomas are typically large tumors at diagnosis. They often have thick, irregular-enhancing margins and a central necrotic core. They are surrounded by vasogenic-type edema, which in fact usually contains infiltration by neoplastic cells. Multifocal disease, which is found in ~20% of cases, is that where multiple areas of enhancement are connected to each other by abnormal white matter signal, which represents microscopic spread to tumor cells. Multicentric disease, on the other hand, is where no such connection can be seen.
How do you diagnose glioblastoma? Tests and procedures used to diagnose glioblastoma include:
- Neurological exam. During a neurological exam, your doctor will ask you about your signs and symptoms.
- Imaging tests. Imaging tests can help your doctor determine the location and size of your brain tumor.
- Removing a sample of tissue for testing (biopsy).
Treatment and prognosis
Biopsy and tumor debulking with postoperative adjuvant radiotherapy and chemotherapy (temozolomide) are the most commonly carried out treatment. Newer therapies include antiangiogenesis (e.g. bevacizumab) and immunotherapy.
In individuals 70 years of age or younger standard Stupp protocol is usual. In older individuals, radiotherapy is usually administered as a shorter course, but even in this setting adding temozolomide significantly increases survival, especially in MGMT methylated (inactive) tumors. Despite this, it carries a poor prognosis with a median survival of fewer than 2 years.
Is there pain with glioblastoma? Headaches and facial pain have been identified as the most prevalent form of pain among patients with glioblastoma multiforme, the most common malignant primary brain tumour.