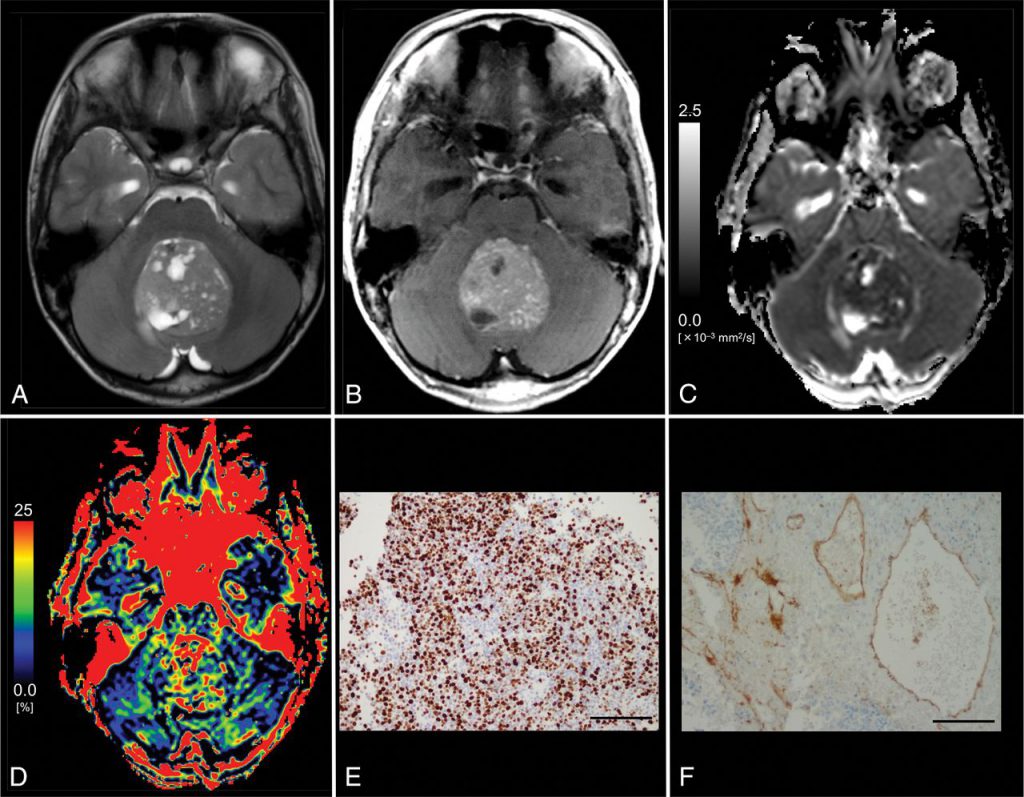
Brain tumors remain a significant health problem in the worldwide. Overall, they comprise some of the most malignant tumors known to affect human beings and are generally refractory to all modalities of treatment.
Angiogenesis underlies all tumor growth by providing oxygen and nutrients to support increased cellular proliferation and metabolism and to remove waste products. However, tumors cannot create their own blood supply and when malignant tumors are very small, they rely principally on diffusion for survival. If a tumor is to grow beyond a few millimeters in size, angiogenesis will be required. In the central nervous system, gliomas are most studied tumors with regard to MR perfusion imaging. In the treatment of brain tumors, surgical intervention remains a common and effective therapeutic option. Recent advances in neuroimaging have provided neurosurgeons with new tools to overcome the challenge of differentiating healthy tissue from tumor-infiltrated tissue, with the aim of increasing the likelihood of maximizing the extent of resection volume while minimizing injury to functionally important regions.
What kind of doctor does brain imaging? Brain imaging helps neurosurgeons diagnose, treat, and monitor people with an array of neurologic issues.
Modalities
- Diffusion
- DTI
- IVIM
- MRS
- DSC
- T1
- T2
- Proton Density imaging
How AI is improving cancer diagnostics? In people with only one scan available, the AI outperformed all of the six radiologists who also examined the CT scans to assess risk of lung cancer. The AI reduced the number of false positives by 11% and false negative by 5%. When there were two scans, the radiologists did about as well as the computer.
Diffusion
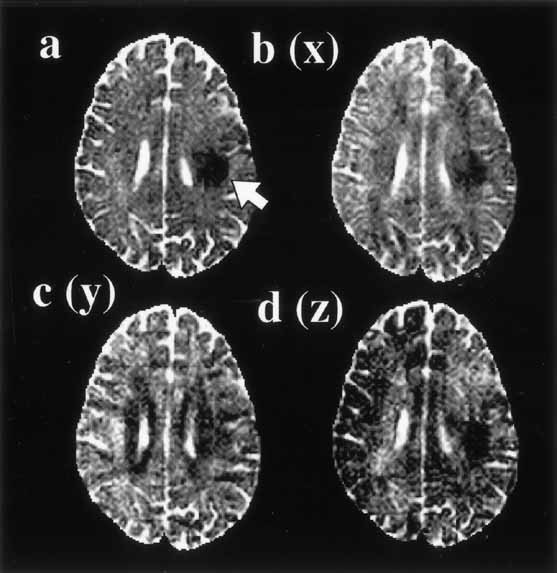
Diffusion imaging, which permits monoexponential analysis and provides directional diffusion information, is performed routinely in brain tumor patients. More advanced methods require improvement in acquisition speed and spatial resolution to gain clinical acceptance. For most clinical applications of diffusion imaging, it is assumed that the diffusion signal vs diffusion weighting factor decays monoexponentially. Within this framework, measurement of a single diffusion coefficient in brain tumors permits an approximate categorization of tumor type and for some tumors definitive diagnosis. In most brain tumors, when compared to normal brain tissue, the diffusion coefficient is elevated.
What does restricted diffusion in the brain mean? For many disorders several processes may act in concert to reduce the ADC. Cerebral Infarction. Restricted diffusion typically occurs within 30-120 minutes after a cerebral infarction, returning to normal by 10-14 days. The principal mechanisms are thought to be: Increase in intracellular water.
Diffusion Tensor Imaging (DTI)
Most of DTI studies in brain tumors focused on the analysis of different parts within the tumor using various DTI metrics. But it is often helpful to measure reactive and infiltrative changes in the tissue surrounding the tumor. DTI measures water diffusion properties of neural tissue and can be used to approximate functionally relevant anatomical white matter tracts in the brain.
Water molecules, the principle components of the brain, are in constant motion caused by random thermal fluctuation. By applying a pair of dephasing and rephrasing magnetic field gradients, MR imaging may be sensitized to the motion (diffusion) in the direction of the field gradient. This gradient pulse configuration is often known as diffusion weighting. Novel applications of diffusion tensor imaging (DTI), and DTI-derived tractography (DDT) have demonstrated that preoperative, non-invasive imaging of eloquent cortical regions and functionally relevant white matter tracts (WMT) is critical during surgical planning to reduce postoperative deficits, which can decrease quality of life and overall survival.
IVIM
Advanced IVIM-model-based analysis of noncontrast multiple b-value DWI data can be used to characterize the diffusivity (Dfast) and perfusion volume (f) of a fast-diffusing tissue component felt to represent the intracapillary water, which differs significantly between low- and high-grade tumors and shows some correlation with capillary blood volume (CBV) from PWI. Whether this will prove to be translatable to clinical use and what advantage it might have over contrast-based methods remains to be seen. IVIM is a new imaging tool and an alternative technique to dynamic susceptibility contrast-MRI which is of considerable interest at present. This is partly because it does not require the use of a contrast agent and relies on the intrinsic properties of motion in the capillaries of the spins. intravoxel incoherent motion is more sensitive to the incoherent motion that originates from small capillary vessels, while the dynamic susceptibility contrast signal is also contaminated by the input from larger arteries and veins, which may result in an overestimation of the blood volume. Although there are limitations due to the heterogeneity of the sample considered in our study, intravoxel incoherent motion has been shown to be an accurate noninvasive radiological biomarker, useful to distinguish between low and high grade glial tumours.
MRS
Magnetic resonance spectroscopy (MRS) is an important modality in evaluating and grading brain tumors. The grading of tumors with MRS had the additional advantage of being a noninvasive diagnostic procedure. MRS is an important modality for the evaluation of tumor type and grade, as well as for targeting and evaluating response to therapy. There are two classes of spatial localization techniques for MR spectroscopy; single-voxel (SV) techniques (commonly used methods) which record spectra from one region of the brain at a time, or multi-voxel techniques ‘MR spectroscopic imaging’ (MRSI), also called ‘Chemical Shift Imaging’ which simultaneously record spectra from multiple regions and thereby map out the spatial distribution of metabolites within the brain. MRSI is typically performed in 2- or 3-dimensions, but does not usually include full brain coverage.
What is an MRS test? Magnetic resonance spectroscopy (MRS), also known as nuclear magnetic resonance (NMR) spectroscopy, is a non-invasive analytical technique that has been used to study metabolic changes in brain tumors, strokes, seizure disorders, Alzheimer’s disease, depression and other diseases affecting the brain.
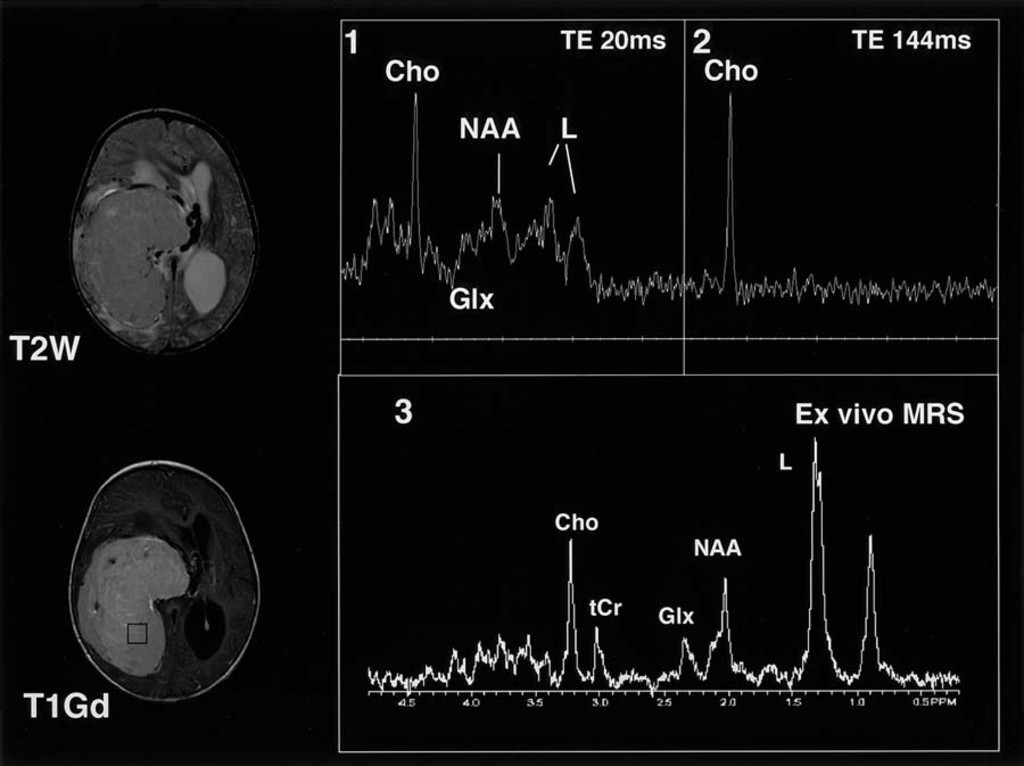
DSC
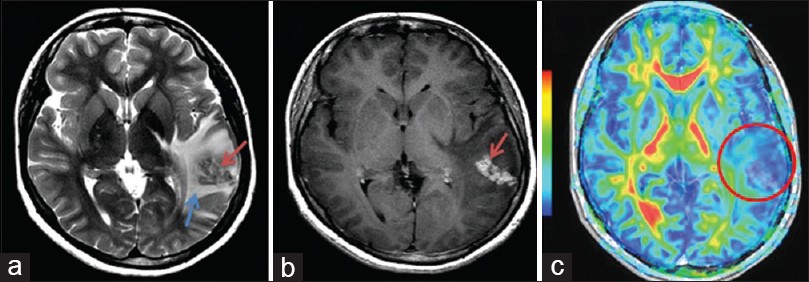
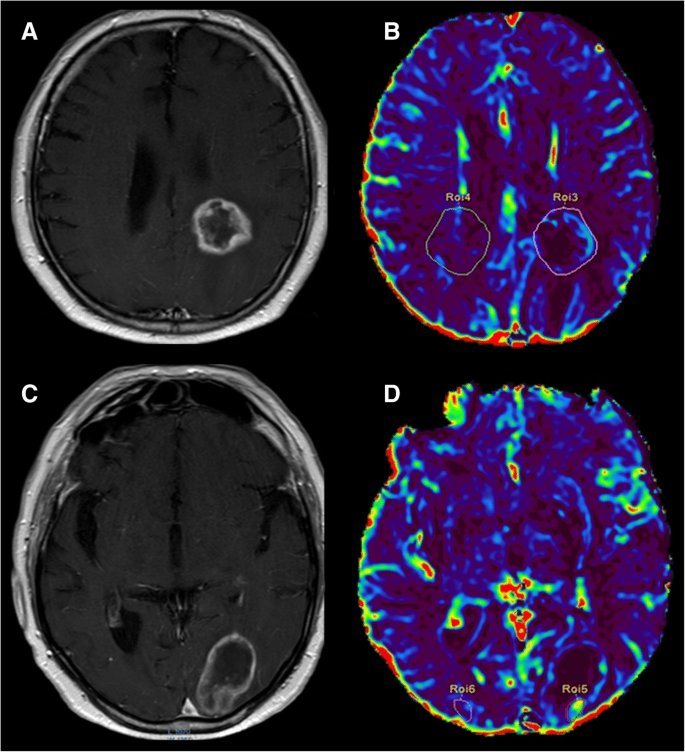
DSC-MRI measurements of tumor rCBV have been widely used in neuro-oncology for glioma grading, prognosis prediction and differentiating recurrent
tumor from radiation necrosis. Although the WHO classification remains the gold standard for prognosis and treatment in brain tumors, DSC-MRI can help predict glioma grade and therefore better direct therapy. DSC-MRI can also help locally guide biopsy and treatment based on the portion of the tumor that appears the most aggressive, which is not necessarily the part of the tumor that most avidly enhances. DSC imaging may be able to predict time to progression in patients with glioblastoma.
What is brain perfusion CT scan? Computed tomography (CT) perfusion of the head uses special x-ray equipment to show which areas of the brain are adequately supplied with blood (perfused) and provides detailed information about blood flow to the brain. CT perfusion is fast, painless, noninvasive and accurate.
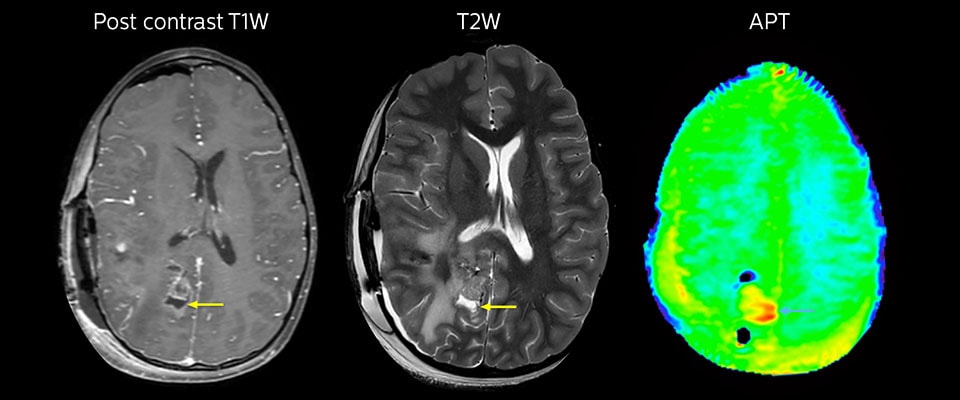
T1
Using the absolute T1 values in the brain before and after intravenous injection of contrast agent, a measurement of contrast agent concentration per voxel of the tumor can be calculated. Dynamic imaging of T1 relaxation time during contrast agent injection can be used to objectively locate these xenograft brain tumors and to track gadolinium-based contrast uptake over time.
The T1 parametric maps made it easy to identify the regions of contrast enhancement and thus tumor location. Doubling the typical human dose of contrast agent resulted in a clearer demarcation of these tumors. Therefore, T1 imaging of brain tumors is gadolinium dose dependent and improves detection of tumors by MRI. The use of T1 maps provides a quantitative means to evaluate tumor detection by gadolinium-based contrast agents over time. This dynamic quantitative T1 imaging technique will also enable future quantitative evaluation of various targeted MRI contrast agents.
What does T1 mean in MRI? T1 (longitudinal relaxation time) is the time constant which determines the rate at which excited protons return to equilibrium. It is a measure of the time taken for spinning protons to realign with the external magnetic field.
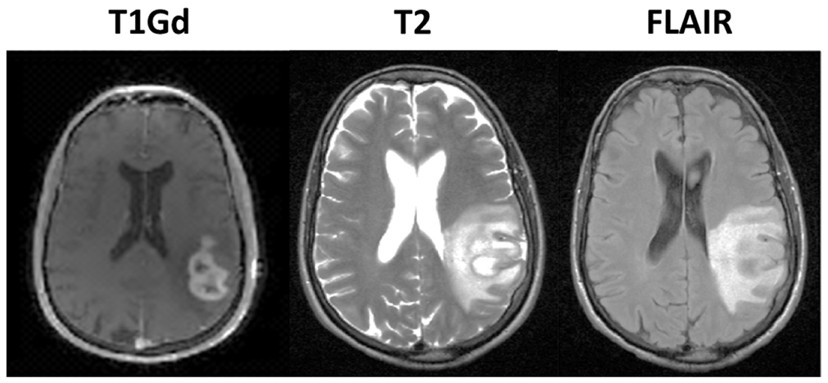
T2
T2 weighted MRI scans are used for the analysis of the diseased brain tissue. The method is based on the fact that pathologic and normal tissues have different T2 relaxation times. T2 images are known as “pathology scans”. It has been shown that measurement of the change in T2 provides important information about the mobility and chemical environment of the tissues of interest. With phantom measurements for a reliable T2 imaging, it is necessary to cover the range of T2 relaxation times of the human brain.
The importance of T2 imaging sequences could be suitable to control the tumor progression under anti-angiogenetic therapy. By use of T2 maps it seems achievable to detect tumor progression by an increase in T2 relaxation times in healthy appearing brain tissue before demarcated changes are visible in usual MRI sequences.
P.D
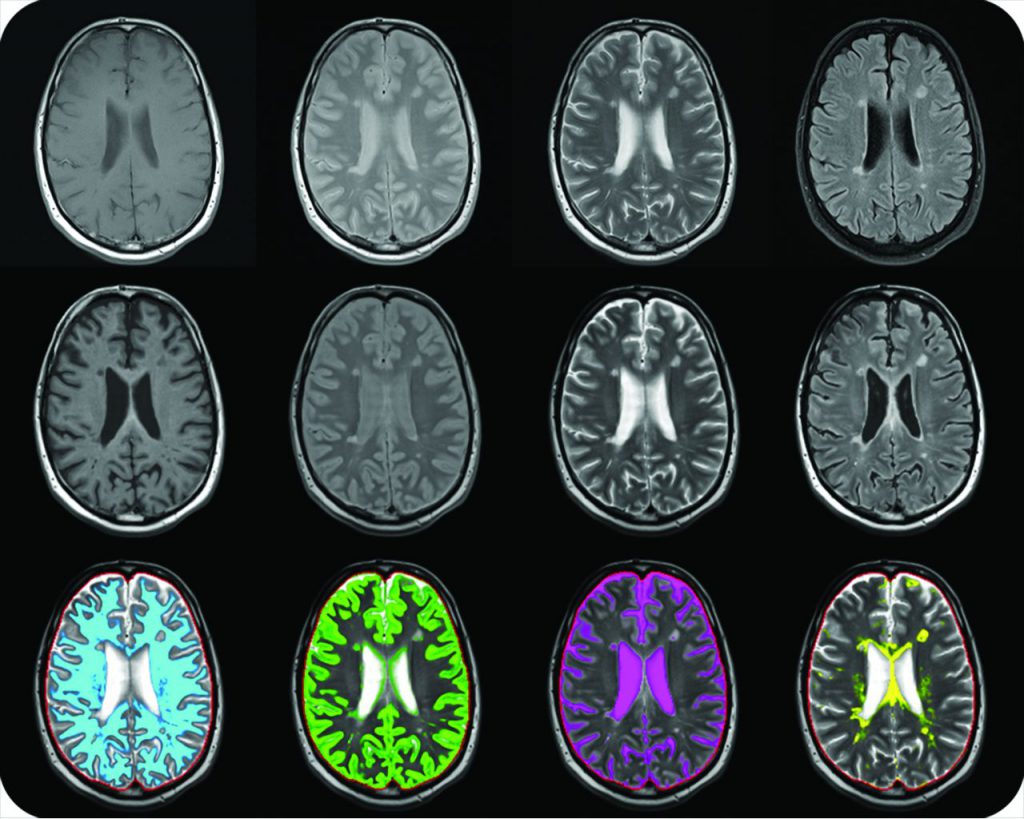
The proton density refers to the number of hydrogen nuclei per unit volume. A region with a greater number of spins will contribute more magnetization and thus more signal than an equivalent region containing fewer spins. Proton density images are designed to show purely this underlying density variation. Proton density images rely on a very long TR value to eliminate T1-weighted contrast and a very short TE value to eliminate T2-weighted contrast. The long TR allows recovery of full equilibrium magnetization for all tissues. The resulting image then reflects the spatial distribution of proton density across the patient, without additional T1- or T2-weighted contrast. PD may be measured and mapped with a variety of pulse sequences. Use of coregistered maps of T1, T2, and PD may be further postprocessed for segmentation and volumetry, generation of distribution histograms, as well as derivation of synthetic MR images. In tumours, PD was found to be higher than normal white matter, although the values did not enable different tumour types to be distinguished, even when used in conjunction with T1 and T2 values. The range of measured normal values for grey matter was large, and poor precision (reproducibility) in the measurement procedure may have contributed to this failure.High correlation between PD and T1, mean that PD did not contribute any extra information.
Machine Learning
The introduction of quantitative image analysis has given rise to fields such as radiomics which have been used to predict clinical sequelae. One growing area of interest for analysis is brain tumors, in particular glioblastoma multiform (GBM). Tumor segmentation is an important step in the pipeline in the analysis of this pathology. Manual segmentation is often inconsistent as it varies between observers. Automated segmentation has been proposed to combat this issue. Methodologies such as convolutional neural networks (CNNs) which are machine learning pipelines modelled on the biological process of neurons (called nodes) and synapses (connections) have been of interest in the literature. We investigate the role of CNNs to segment brain tumors by firstly taking an educational look at CNNs and perform a literature search to determine an example pipeline for segmentation. We then investigate the future use of CNNs by exploring a novel field—radiomics. With the introduction of methods to quantitatively analyse gliomas with computational methods comes a new frontier for radiology. It is important for radiologists to be abreast of advances in machine learning. Convolutional neural networks are a unique machine learning structure originally modelled on the human visual cortex. The brain was studied due to the abundance of segmentation methods that are already available and well established in the literature. Machine learning is fast developing and is exponentially being represented at international conferences. An educational perspective is needed for radiologists. The Machine learning algorithms are used for classification of MR brain image either as normal or abnormal. The major aim of ML algorithms is to automatically learn and make intelligent decisions. Using machine learning techniques that learn the pattern of brain tumor is useful because manual segmentation is time-consuming and being susceptible to human errors or mistakes.
T2 (transverse relaxation time) is the time constant which determines the rate at which excited protons reach equilibrium or go out of phase with each other. It is a measure of the time taken for spinning protons to lose phase coherence among the nuclei spinning perpendicular to the main field.
Proton-density weighted images are related to the number of nuclei in the area being imaged (number of hydrogen protons), as opposed to the magnetic characteristics of the hydrogen nuclei. They are produced from the first echo. PD weighted images result when the contribution of both T1 and T2 contrast is minimized.
Image classification is a supervised learning problem: define a set of target classes (objects to identify in images), and train a model to recognize them using labeled example photos. Early computer vision models relied on raw pixel data as the input to the model.

