Alzheimer’s imaging
Alzheimer’s (Dementia) is an acquired syndrome in which a person suffers from progressive impairment in one of the following six cognitive domains: complex attention, executive function, learning and memory, language, perceptual-motor function, and/or social cognition. These cognitive deficits cannot be attributed to another mental disorder (e.g., depression or schizophrenia) and must be severe enough to interfere with independent daily functioning to be classified as a “major neurocognitive disorder” (major NCD) according to the DSM-V edition. This new choice in terminology in the DSM-V was chosen in order to be able to also define a “mild neurocognitive disorder” (equivalent to mild cognitive impairment (MCI) and/or prodromal dementia), with the key distinction that persons with mild NCD remain independent in daily functioning. This shift to diagnose NCD as early as possible stems from the understanding that these diseases have a long prodromal phase, in which early diagnosis and early treatment may have beneficial effects. Importantly, mild NCD is not always a precursor to major NCD (dementia), and the diagnosis does not require further decline. In the assessment of patients suspected for dementia, neuroimaging plays an important role in establishing the diagnosis and subtyping the underlying cause of the cognitive impairment.
Can you see Alzheimer’s on MRI? MRI can detect brain abnormalities associated with mild cognitive impairment (MCI) and can be used to predict which patients with MCI may eventually develop Alzheimer’s disease. In the early stages of Alzheimer’s disease, an MRI scan of the brain may be normal.
Alzheimer’s: Role of Neuroimaging
Neuroimaging is one of the most important ancillary tests in the clinical work-up of a patient suspected of dementia. The primary aim of imaging used to be the exclusion of treatable causes of cognitive impairment (such as subdural hematomas, brain tumors, or normal pressure hydrocephalus), but imaging is increasingly helpful in the identification of underlying causes of dementia. Though for the majority of diseases causing dementia, no specific imaging criteria have been formulated in the clinical diagnostic criteria, it is notable that more recent revisions of criteria are increasingly including imaging (for positive as well as negative predictive value).
Can dementia be seen on an MRI? MRI can be used to rule out other causes, find characteristic patterns of brain damage, and differentiate between types of dementia. Brain scans do not always show abnormalities in people diagnosed with dementia, as sometimes there are no visible changes in the brain.
Alzheimer’s imaging Modalities
- Volumetry:
Brain volumes computed from magnetic resonance images have potential for assisting with the diagnosis of individual dementia patients, provided that they have low measurement error and high reliability. A good compliance with volumetric calculations has been reported. The assessment is performed on coronal or 3D T1 weighted images, on MRI or coronal CT, with an angulation along the dorsal border of the brainstem or perpendicular to the anterior commissure–posterior commissure (AC-PC). A visual estimation of the height of hippocampus, the width of the temporal horn of lateral ventricle and the width of choroid fissure, creates a 5-grade visual rating scale.
Manual outlining has for a long time been considered as the most accurate way of measuring the volume of different structures in the brain and it is still regarded as the golden standard. A manual evaluation can be performed in several ways and the most common form is to draw a line around the region of interest in all the contiguous slices where the region can be observed in the 3D MR image. Other methods, such as stereological point counting techniques, can also be used.
What scan is used to detect Alzheimer’s? A brain scan—using either computed tomography (CT) or magnetic resonance imaging (MRI)—is generally included in the standard evaluation for Alzheimer’s disease and other forms of dementia.
- Perfusion (ASL):
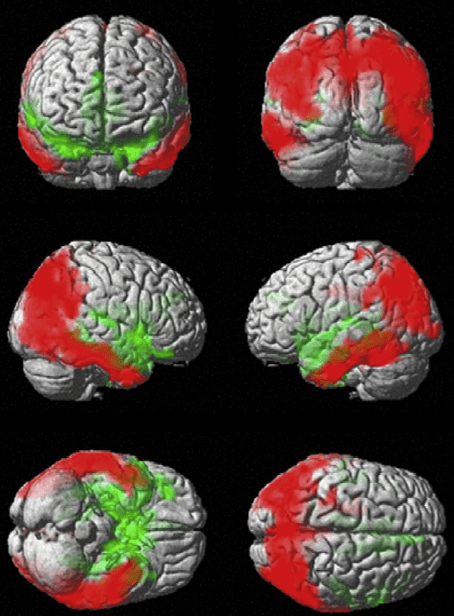
ASL PWI uses inflowing water molecules in blood as an endogenous contrast agent. This ‘‘magnetic label’’ is delivered to the slice of interest and alters the image contrast at a rate determined by the local CBF. Typically, two images are acquired and the CBF map is produced by subtracting the magnetic labeling image from the control image. According to the labeling method, ASL can be divided into sub-classes, namely continuous, pulsed, and velocity-selective ASL. Studies have been conducted to enhance ASL labeling optimization with tagging schemas for improved labeling efficiency. ASL studies have generally demonstrated significant hypoperfusion in various brain regions in clinical AD. These included temporal, parietal, and frontal lobes, as well as the PCC, which may not always match atrophy. Perfusion insufficiency has been repeatedly proved to be correlated with the severity of the disease. For example, one of the early ASL studies reported that reductions of the whole brain CBF and the rCBF in the posterior parietal and PCC in AD patients were correlated with their cognitive assessment scores. A recent ASL perfusion study has been able to detect a quantitatively significant decrease of the absolute rCBF in the precuneus and PCC in AD patients compared with matched healthy controls.
Which imaging technique is specific for Alzheimer disease? A PET/CT scan can help differentiate Alzheimer’s disease from other types of dementia. Another nuclear medicine test called a single-photon emission computed tomography (SPECT) scan is also used for this purpose.
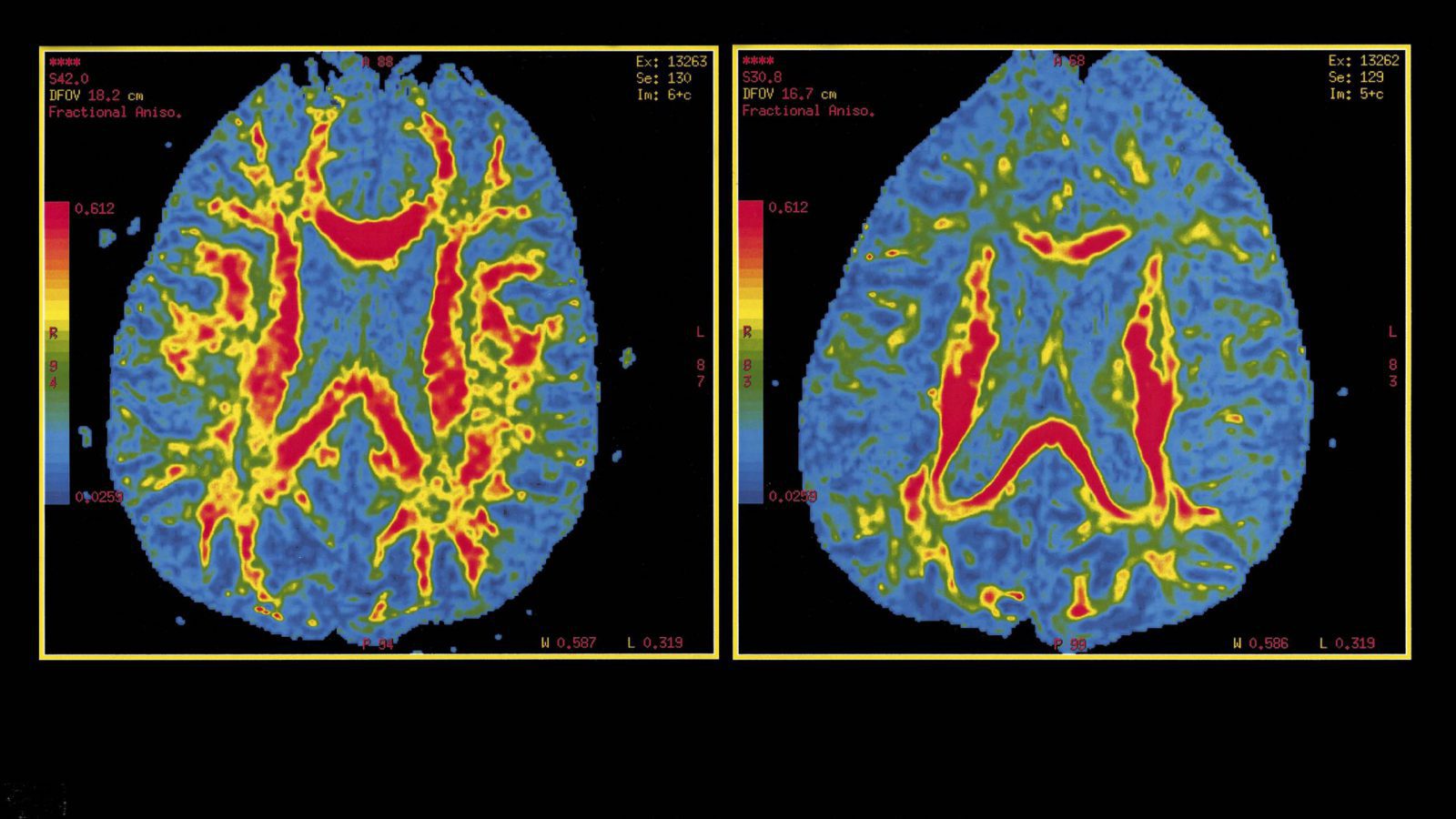
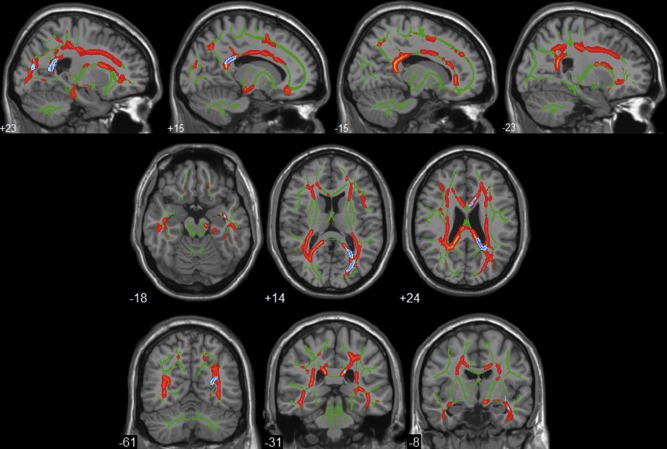
3. DTI:
DTI is one of the most effective MR tools for the investigation of the brain anatomy. In addition to the gray matter, histopathological studies indicate that white matter is also a good target for both the early diagnosis of AD and for monitoring disease progression, which motivates us to use DTI to study AD patients in vivo. The main DTI measures, apparent diffusion coefficient, and provide a direct assessment of WM fibers and may be used as a new biomarker for AD. These findings were found to correlate with cognitive assessments, rates of AD progression and were also able to differentiate among groups including mild cognitive impairment, AD, and other dementias. Because MR scanners are widely available, non-invasive, and reliable there is increasing interest in using DTI for diagnosing AD. The relevance of AD, clinical evaluation difficulties, weakness in current diagnostic criteria, the urgent need for early AD diagnosis and limited DTI usage in this clinical context, is the main reasons for reviewing the concepts of DTI and its clinical applicability to AD management. DTI analyses in AD or MCI have demonstrated brain structural disturbances predominately in regions commonly affected in early AD including the hippocampal area, temporal area, posterior cingulate, and corpus callosum.
How do you confirm Alzheimer’s disease? It’s important to note that Alzheimer’s disease can be definitively diagnosed only after death, by linking clinical measures with an examination of brain tissue in an autopsy. Occasionally, biomarkers—measures of what is happening inside the living body—are used to diagnose Alzheimer’s.
- fMRI
fMR(rest)
Connectivity in resting-state functional magnetic resonance imaging (rsfMRI) is an emerging AD biomarker that holds promise for early diagnosis. RsfMRI indirectly measures neural processing in the brain using blood oxygenation and can be used to identify spatially distributed networks. The National Institute on Aging–Alzheimer’s Association lists rsfMRI functional connectivity as a potential biomarker of neuronal injury, at an early stage of validation. The existing literature is indeed mostly composed of proof-of-concept cross-sectional comparisons of cognitively healthy elderly individuals with patients suffering from mild (MCI) or severe (dementia) AD symptoms. rs-fMRI have shown a disruption in functional connectivity (FC) between structures that are part of the default-mode network (DMN) at an early stage of AD and mild cognitive impairment (MCI). rs-fMRI is a promising technique that can identify functional impairment in early Alzheimer’s disease, and bridge the gap between molecular pathology (amyloid PET) and frank neurodegeneration with tissue loss (atrophy on MRI).
fMR(task)
Right hippocampal magnitude may be the most promising of these candidate measures in a leveraged context. These initial estimates of test-retest reliability and power justify evaluation of encoding-task fMRI as a potential biomarker for signal of effect in exploratory and proof-of-concept trials in mild AD. Validation of these results with larger sample sizes and assessment in multisite studies is warranted. Task-fMRI may potentially help detect a signal of effect and guide early-development programs for novel AD therapeutics.
What part of the brain is linked to Alzheimer’s? At first, Alzheimer’s disease typically destroys neurons and their connections in parts of the brain involved in memory, including the entorhinal cortex and hippocampus. It later affects areas in the cerebral cortex responsible for language, reasoning, and social behavior.
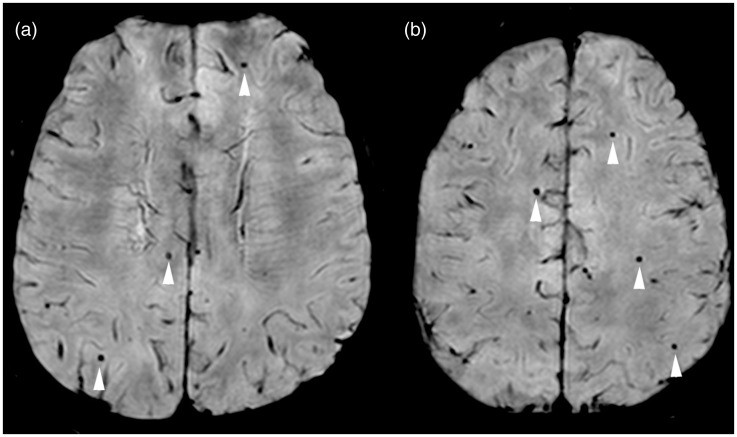
- Susceptibility-Weighted Imaging (SWI)
SWI revealed more extensive brain microhemorrhages than standard MRI techniques, allowing the radiologic diagnosis of cerebral amyloid angiopathy. SWI shows promise as a more sensitive diagnostic tool than standard brain MRI for the evaluation of patients with dementia. SWI MR brain imaging may have significant clinical application. The differential diagnosis of microhemorrhages would include hypertension, trauma and cavernomas. Cerebral amyloid angiopathy with microhemorrhages is known to be prevalent in patients with Alzheimer’s disease, but may be unrecognized on clinical evaluation and standard brain imaging. The demonstration of more numerous microhemorrhages on SWI, as compared to GE-T2*, may represent a more sensitive method for detecting cerebral amyloid angiopathy as a contributing factor to the patient’s dementia. Systematic and sequential studies using SWI to evaluate mild cognitive impairment and dementia may help elucidate the apparent relationship of cerebral amyloid angiopathy and microhemorrhages to Alzheimer’s disease.
SWI may have significant utility in the clinical management of patients with dementia and Alzheimer’s disease as well as significant research applications to define the role of cerebral amyloid angiopathy and microhemorrhages in the pathophysiology of dementia.
How do Alzheimer patients feel? Eventually, much of what we consider conscious thought disappears. But emotional aspects of the disease may be just as important, especially to the friends and family who serve as caregivers. On the negative side, Alzheimer’s sufferers may have feelings of anger, anxiety, depression, fear, and loneliness.
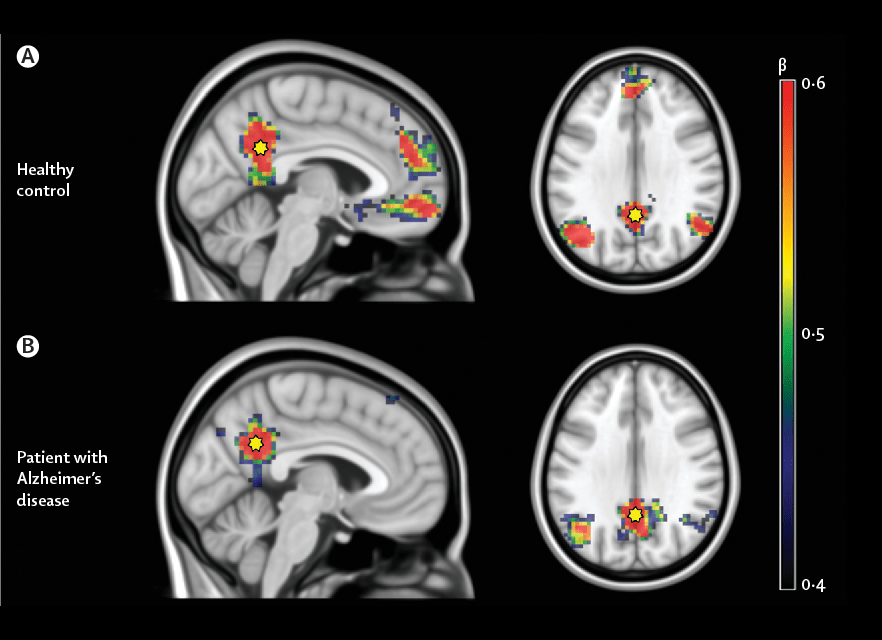
Machine Learning
Most approaches to machine learning from electronic health data can only predict a single endpoint. The ability to simultaneously simulate dozens of patient characteristics is a crucial step towards personalized medicine for Alzheimer’s disease. Processing large quantities of Alzheimer imaging is not an easy task, and researchers have turned their attention towards machine learning, a set of computer algorithms that automatically adapt their output towards the intended goal. Since AD is a complex disease showing heterogenous structural and functional changes at brain level, these techniques can lead to a deeper understanding of new aspects of AD progression. As a matter of fact, ML approaches are particularly sensitive to distributed disease-specific changes observed in many human structural and functional imaging studies. They are designed to identify patterns in data that differentiate between several classes. ML classification offers powerful prediction methods for the disease state of an individual.
What is the main cause of Alzheimer’s? Scientists don’t yet fully understand what causes Alzheimer’s disease in most people. The causes probably include a combination of age-related changes in the brain, along with genetic, environmental, and lifestyle factors.